Protein misfolding neurodegenerative diseases
Protein misfolding neurodegenerative diseases include prion diseases, which affect humans and animals, and human-specific conditions such as Alzheimer's disease, Parkinson's disease, Huntington's disease and motor neuron disease. These diseases are invariably fatal and arise through neurotoxicity induced by the misfolding, aggregation, and accumulation in the brain of disease specific host proteins. Prion diseases are an important paradigm for neurodegenerative diseases in general, since Alzheimer’s, Parkinson’s and motor neuron disease, and tauopathies, show prion-like phenomena, such as cell-to-cell spread of misfolded protein.
Neurodegenerative diseases that arise as a consequence of protein misfolding are an important issue for both animal and human health. Prion diseases are a threat to human food safety since they are transmissible between individuals of the same or different species. This threat has been evidenced by the outbreak of BSE in cattle and subsequent emergence of variant CJD in humans, which is believed to be due to the consumption of BSE-contaminated bovine products. Furthermore, the increasing number of aged people with their predisposition to neurodegeneration has led to an increase in the number of humans with conditions such as Alzheimer's and Parkinson's disease. For these reasons, protein misfolding neurodegenerative diseases are a challenge to society. Understanding the mechanism of neurotoxicity induced by protein misfolding will allow the design of strategies to alleviate their burden.
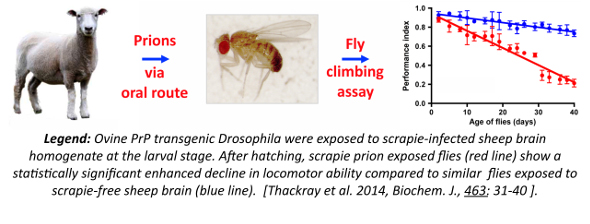
The study of protein misfolding-induced neurotoxicity in the natural host is difficult and research in this area relies upon the use of animal models. We have modelled transmissible mammalian prion disease in the invertebrate host Drosophila melanogaster in order to provide a new experimental host to study protein misfolding neurodegenerative disease. Modelling prion disease in a genetically well-defined tractable invertebrate host will provide a novel approach to identify the genes and cellular mechanisms that affect the way misfolded and aggregated prion protein causes neurotoxicity and neurodegeneration. Understanding this process will allow the design of better diagnostic tests for these conditions. In addition, knowledge of the mechanism of this process will allow the development of potential therapeutic strategies for prion diseases per se and human neurodegenerative diseases that show prion-like phenomena. Furthermore, the use of an invertebrate host such as Drosophila for these studies will contribute to the principles of the 3Rs approach to reduce, replace and refine the use of sentient animals in research.